Ultimate Guide to Anodic vs. Cathodic Protection: Which is Better for Corrosion Control?
Corrosion is a major challenge in industries that rely on metal structures, especially in harsh environments like marine, oil, gas, and chemical industries. To combat this, two of the most effective methods of corrosion prevention are anodic protection and cathodic protection. But how do these methods differ, and which is the right choice for your project? Let’s dive into the ultimate guide on the difference between anodic and cathodic protection.
What is Anodic Protection and How Does it Work?
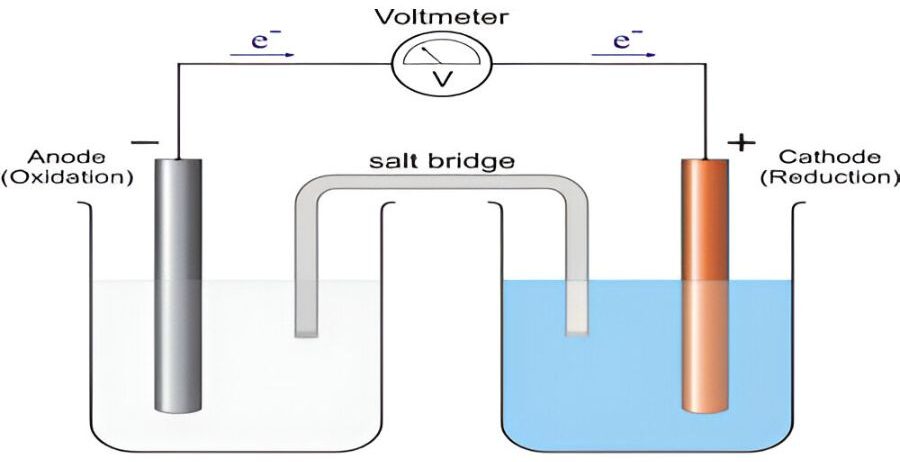
Anodic protection is a corrosion control method where a metal surface is made to act as an anode in an electrochemical cell. The metal is connected to an external power source, which applies a controlled voltage to keep the metal in a passive state. This passive state minimizes the corrosion rate of the metal by forming a protective oxide layer on the surface.
Anodic protection is typically used for metals that have the ability to form a passive film, such as stainless steel and certain carbon steels, in environments like sulfuric acid tanks or chemical storage. The primary goal is to maintain a protective layer that resists corrosion, which is achieved by fine-tuning the voltage applied to the metal.
Key Features of Anodic Protection:
- Used for metals that naturally form passive layers.
- Requires an external power source.
- Effective in environments like chemical storage tanks.
- Prevents uniform and localized corrosion.
What is Cathodic Protection and How Does it Work?
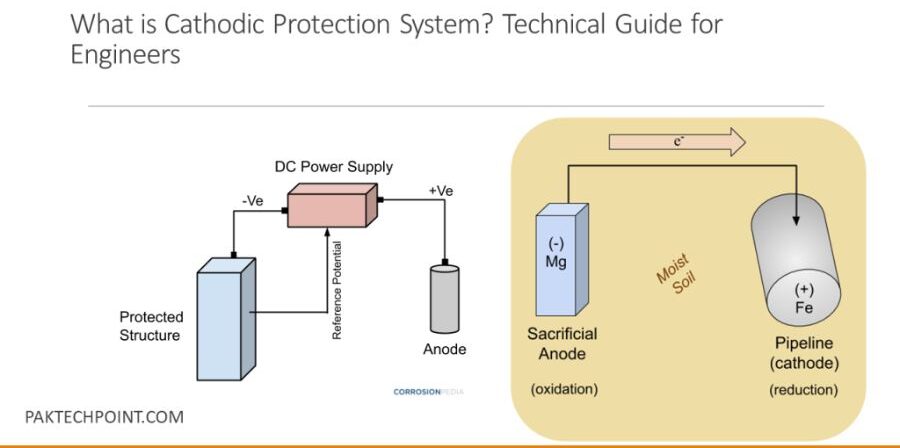
Cathodic protection is a corrosion prevention method that turns a metal surface into a cathode, thereby preventing it from corroding. This is achieved by either using a sacrificial anode or an external power source to provide a flow of electrons to the protected metal, neutralizing corrosion.
There are two main types of cathodic protection:
- Sacrificial Anode Cathodic Protection (SACP): In this method, a more reactive metal, such as magnesium or zinc, is attached to the structure that needs protection. The sacrificial anode corrodes in place of the protected metal, sacrificing itself to keep the main structure safe.
- Impressed Current Cathodic Protection (ICCP): This method uses an external power source to deliver a current to the protected structure. This system is used for large structures like pipelines, oil rigs, and underground tanks.
Key Features of Cathodic Protection:
- It can be used for a wide variety of metals.
- Available in two forms: sacrificial anode and impressed current.
- Widely used in pipelines, marine structures, and underground tanks.
- Effective in preventing pitting and uniform corrosion.
What is the Key Difference Between Anodic and Cathodic Protection?
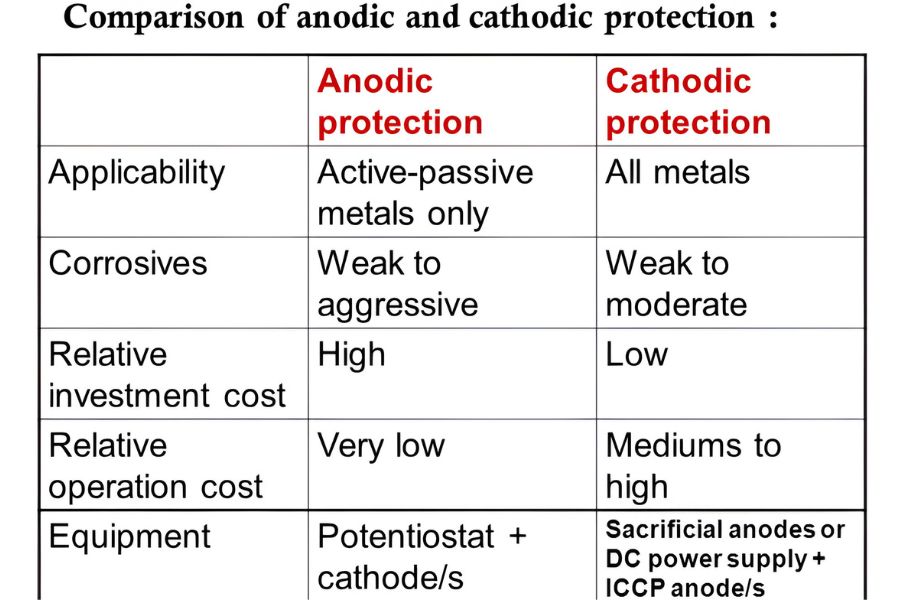
While both methods prevent corrosion, the core difference Between Anodic and Cathodic Protection lies in how they treat the metal surface:
- Anodic Protection: In anodic protection, the metal becomes an anode. It works by maintaining the metal in a passive state, preventing corrosion through the formation of a protective oxide layer. It is ideal for metals like stainless steel in environments where a passive film can form.
- Cathodic Protection: In cathodic protection, the metal becomes a cathode. It works by supplying electrons to the metal, effectively stopping the corrosion process. Cathodic protection is more versatile and can be applied to a variety of metals, including steel and iron, used in large structures like pipelines or underground tanks.
When Should You Choose Anodic Protection Over Cathodic Protection?
Anodic protection should be used when dealing with metals that have the ability to form a passive oxide layer, such as stainless steel in environments like acidic storage tanks. This method is highly effective but requires constant monitoring of the voltage applied to ensure the metal stays in its passive state.
Ideal Conditions for Anodic Protection:
- Presence of highly corrosive environments like chemical tanks.
- Metals like stainless steel form a stable passive layer.
- Systems where the environment is stable and controlled.
When is Cathodic Protection the Best Choice?
Cathodic protection is a more widely used method because of its flexibility and effectiveness in protecting various types of metals, even in aggressive environments. It is especially valuable in large structures like pipelines, ships, and underground storage tanks, where it’s crucial to prevent long-term corrosion damage.
Ideal Conditions for Cathodic Protection:
- Large, metallic structures like pipelines, ships, and offshore platforms.
- Underground or submerged environments where corrosion risk is high.
- Metals like steel, iron, and aluminium.
What Are the Pros and Cons of Anodic and Cathodic Protection?
Anodic Protection Pros:
- Effective for passive metals.
- Provides long-term protection in specific environments.
Anodic Protection Cons:
- Requires constant power supply and monitoring.
- Limited to specific types of metals and environments.
Cathodic Protection Pros:
- It can be used on a wide range of metals.
- Sacrificial anodes are easy to replace when consumed.
- Provides reliable protection in harsh environments.
Cathodic Protection Cons:
- Sacrificial anodes need regular replacement (for SACP).
- ICCP systems require an external power source.
FAQs: Frequently Asked Questions
What is anodic protection?
Anodic protection is a corrosion control method where a metal is made to act as an anode in an electrochemical cell, creating a passive layer to prevent corrosion.
How does cathodic protection work?
Cathodic protection prevents corrosion by making the metal surface a cathode. It uses either a sacrificial anode or an external power source to stop the metal from corroding.
What is the main difference between anodic and cathodic protection?
Anodic protection forms a passive layer on the metal surface, while cathodic protection supplies electrons to the metal, stopping corrosion altogether.
When should anodic protection be used?
Anodic protection is best for metals that naturally form a passive oxide layer, like stainless steel, in stable and controlled environments such as acidic storage tanks.
When is cathodic protection the better choice?
Cathodic protection is ideal for large structures like pipelines, ships, and tanks, especially in harsh environments where long-term corrosion control is crucial.
Closing Insights: Anodic vs Cathodic Protection
When deciding between anodic and cathodic protection, it all comes down to the type of metal, the environment it’s exposed to, and the structure’s size and complexity. If you’re dealing with passive metals in a controlled environment, anodic protection might be the better choice. However, for large structures exposed to harsh environments, cathodic protection is often the most reliable and versatile solution.
No matter which method you choose, both anodic and cathodic protection play a critical role in extending the lifespan of metal structures and minimizing costly repairs due to corrosion.
For expert guidance on corrosion control and training in advanced protection techniques, CORCON – Institute of Corrosion offers specialized programs to equip professionals with the skills needed to tackle corrosion challenges in diverse industries.
Image Reference : Freepik
Disclaimer: All trademarks, logos, and brand names are the property of their respective owners. All company, product, and service names used in this website are for identification purposes only. Use of these names, trademarks, and brands does not imply endorsement.



